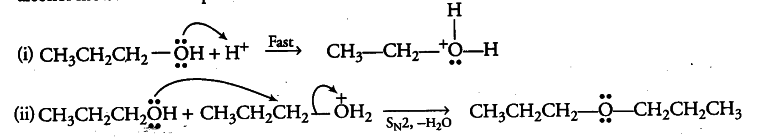Acid catalysed dehydration of 1° alcohols to ethers occurs by reaction involving nucleophilic attack by the alcohol molecule on the protonated alcohol molecule.
Under these conditions, 2° and 3° alcohols, however give alkenes rather than ethers. The reason is that due to steric hindrance, nucleophilic attack by the alcohol molecule on the protonated alcohol molecule does not occur. Instead protonated 2° and 3° alcohols lose a water molecule to form stable 2° and 3° carbocations. These carbocations prefer to lose a proton to form alkenes rather than undergoing nucleophilic attack by alcohol molecule to form ethers.