The metal ions with partially or incomplete filled d-orbitals will be coloured in aqueous solution. While the metal ions having either empty or completely filled d-orbitals are colourless. The colour will be due to d-d transition of electrons. Thus, the outer electronic configuration of metal ions are
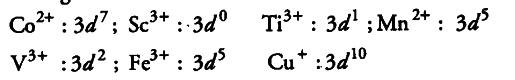
Hence, among the given ions, {{Ti}^{3+}}, {{V}^{3+}}, {{Mn}^{2+}}, {{Fe}^{3+}} and {{CO}^{2+}} will exhibit colour in aqueous solution. While {{Cu}^{+}} and {{Sc}^{3+}} will be colourless.