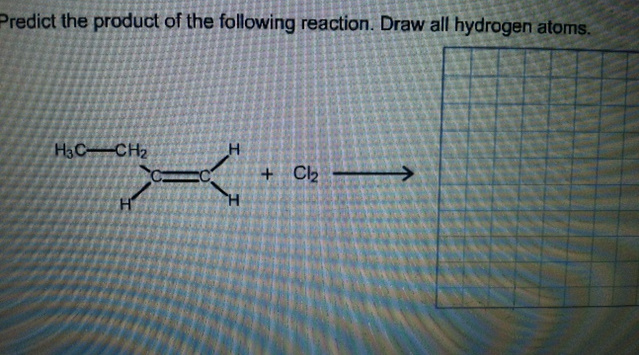
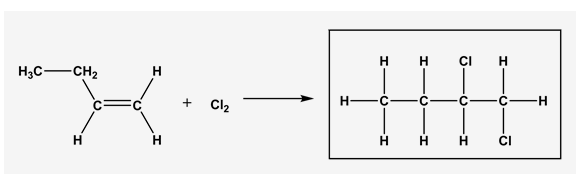
Predict the product of the following reaction. Draw all hydrogen atoms.
Concepts and reason
The addition of halogens in ordinary temperature, in the absence of exposure to UV light to yield vicinal dihalides.
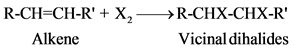
This reaction is proceeded through an ionic mechanism which involves two steps.
Fundamentals
General mechanism of the halogenation reaction:-
Step 1
As the halogen molecules ![]() approach the π-electrons of the alkene, they gets polarized. The polarized halogen molecule undergoes a heterolytic cleavage, reacting as a positive halogen ion to the alkene and forms a cyclic halonium ion.
approach the π-electrons of the alkene, they gets polarized. The polarized halogen molecule undergoes a heterolytic cleavage, reacting as a positive halogen ion to the alkene and forms a cyclic halonium ion.
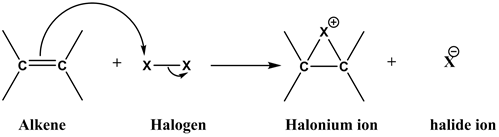
Step 2
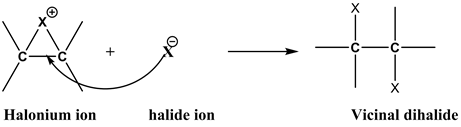
The nucleophilic attack by the halide ion formed in step 1 on the halonium ion from the side opposite to that of the bulky halogen portion causes the opening of the three-member ring and the formation of the vicinal dihalides.
Answer:
Heterolytic cleavage of ![]()
![]()
Formation of halonium ion,
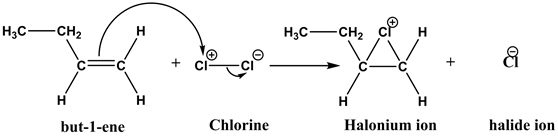
Explanation:
As the chlorine molecules approach the π-electrons of the alkene, they are polarized. Then, the chlorine molecules undergo a heterolytic cleavage and react with an alkene to form cyclic halonium ion and chloride ion.
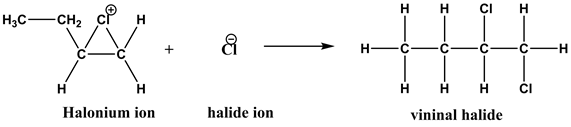
Explanation:
The nucleophilic attack by the chloride ion formed in step 1 on the halonium ion from the side opposite to that of the bulky chlorine portion causes the opening of the three-membered ring and the formation of vicinal dihalide. The addition is trans under these conditions.