Potassium hydroxide and phosphoric acid react to form potassium phosphate and water according to the equation:
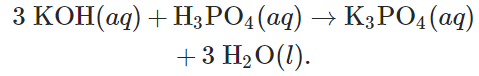
Determine the starting mass of each reactant if 55.7gK3PO4 is produced and 89.8gH3PO4 remains unreacted.
Potassium hydroxide and phosphoric acid react to form potassium phosphate and water according to the equation:
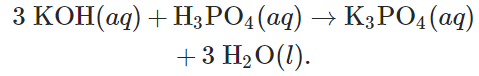
Determine the starting mass of each reactant if 55.7gK3PO4 is produced and 89.8gH3PO4 remains unreacted.