Please explain sp hybridisation in C2H2(ethyne)
- When one s and one p orbital belonging to the same main shell of an atom mix together to form two new equivalent orbitals, the type of hybridization is called sp hybridization. The new orbitals formed are called sp hybrid orbitals.
- They are collinear with an angle of 1800.
- Each of the hybrid orbitals formed has 50% s-character and 50% p-character.
- The remaining two p-orbitals which do not participate in hybridization remain as such.
- If these are half-filled, they may form bonds with other atoms having half-filled atomic orbitals.
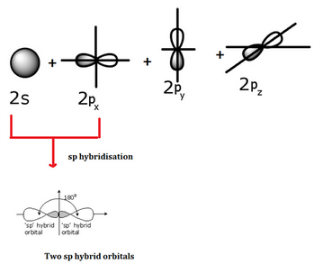
Thus, sp- hybridization arises when one s and one p orbital combine to form two sp-orbital with 180° bond angle and linear shape to the molecule. The percentage of s and p are 50 %. Example: C2H2 (acetylene or ethyne).