Please explain cleansing action of soap and detergents
- Soaps are cleansing agents capable of reacting with water and dislodging the unwanted particles from clothes or skin.
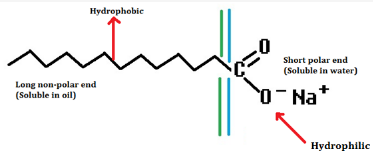
- The molecules of soap are sodium or potassium salts of long chain carboxylic acids.
- A soap molecule has a tadpole shaped structure.
- At one end (long non-polar end) of soap molecule ¡s a hydrocarbon chain which is insoluble ¡n water but soluble in oil.
- At the other end (short polar end) of soap molecule, there is a carboxylate ion which is hydrophilic i.e. waler soluble but insoluble in oil.
- Soap on mixing with water forms a concentrated solution and causes foaming.
- The long non-polar end of soap gravitates towards and surrounds the dirt and absorbs the dust in it
- The short polar end with the carboxylate ion repels the water away from the dirt.
- A spherical aggregate of soap molecules is formed in the soap solution in wate and is called a micelle.
- Thus, the soap molecule dissolves the dirt and our clothes get clean.