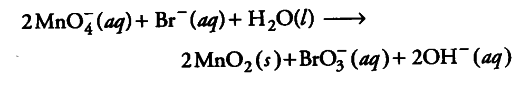Permanganate ion reacts with bromide ion in basic medium to give manganese dioxide and bromate ion. Write the balanced ionic equation for the reaction.
Step 1: The skeletal ionic equation is
![]()
Step 2: Assign oxidation numbers for Mn and Br
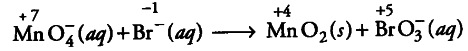
this indicates that permanganate ion is the oxidant and bromide ion is the reductant.
Step 3: Calculate the increase and decrease of oxidation number, and make the increase equal to the decrease.

Step 4: As the reaction occurs in the basic medium and the ionic charges are not equal on both sides, add 2 OH ions on the right to make ionic charges equal.
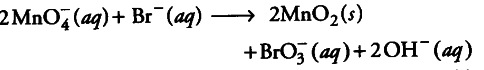
Step 5 Finally, count the hydrogen atoms and add appropriate number of water molecules (i.e., one ${ H }_{ 2 }$0 molecule) on the left side to achieve balanced redox change).