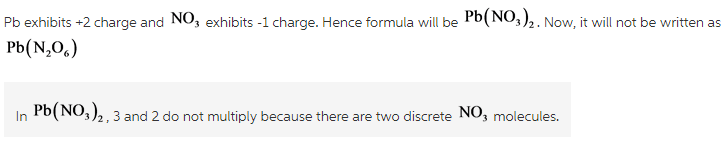
Pb(NO3)2
I understand the ion charge of NO3 to be -1
My chem professor gave us a sheet with common monatomic cation/anion charges on it and it has Pb+4 and Pb+2. In the book, for this particular problem it uses +2 and says it used that charge because of the 2 subscript in Pb(NO3)2, which i understand, but why does the 3 and 2 not mult. together and give you a 6 like it would if you were balancing an equation?
Concepts and reason
The cations and anions are not always atoms, they can be molecules also. That is, molecules can also exhibit positive or negative charge.
Fundamentals
Molecular anions like ![]() etc exhibits charge on the molecule and when they react with cation, they still exist as a single molecule.
etc exhibits charge on the molecule and when they react with cation, they still exist as a single molecule.
Answer:
The molecule, ![]() exhibits a unit negative charge.
exhibits a unit negative charge.
Explanation:
Here N possess +5 charge and 3 O possess 3(-2) = -6 charge. Hence, +5-6 = -1 charge remains on the molecule.
Eplanation: