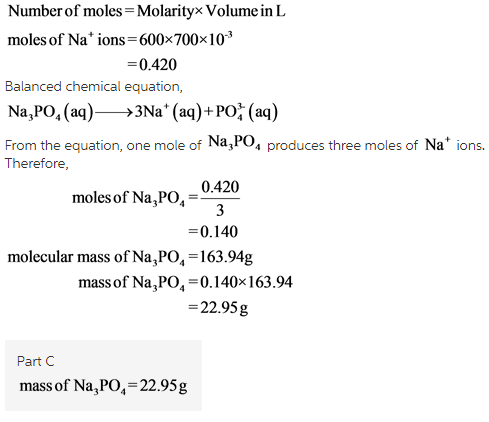Part A: What is the concentration of K^+ in 0.15 M of K2S ?
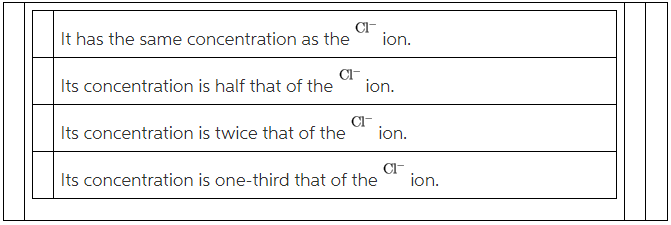
Part B: If \rm CaCl_2 is dissolved in water, what can be said about the concentration of the Ca^{2+} ion?
Part C: A scientist wants to make a solution of tribasic sodium phosphate, \rm Na_3PO_4, for a laboratory experiment. How many grams of \rm Na_3PO_4 will be needed to produce 700 mL of a solution that has a concentration of \rm Na^+ ions of 0.600 \it M?
Express your answer numerically in grams.
Answer:
Concepts and reason
A balance chemical equation of a reaction is the simple representation of the reaction, which gives the idea of the particles and participates in the reaction. Concentration is the amount of substance present in a given volume of a solution.
Mole is the mass that explains the particles present in the substance.
Fundamentals
A balance chemical equation of a reaction provides the number of particles and participates in a chemical reaction.
The molarity of a solution is the number of moles of the solute present in 1L of the solution.
Mole is the mass that explains the particles present in the substance. A mole can be determined by using given molarity and volume.

Answer:
(A)
Balanced chemical equation of the reaction is
![]()
From the chemical equation, one mole of ![]() produces two moles of
produces two moles of ![]() .
.
Therefore,

(B)
Balanced chemical equation,
![]()
From the equation,
![]()
Therefore, concentration of the ![]() ion is half of the concentration of
ion is half of the concentration of ![]() ion.
ion.

( c)