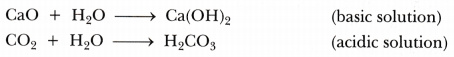On heating, calcium carbonate gets converted into calcium oxide and carbon dioxide.
- Is this a physical or a chemical change?
- Can you prepare one acidic and one basic solution by using the products formed in the
above process? If so, write the chemical equation involved.
Answer:
- Chemical change.
- Acidic and basic solutions can be prepared by dissolving the products of the above process in water.