Nitrogen (atomic no. 7) and phosphorous (atomic no. 15) belong to group 15 of the Periodic Table. Write the electronic configuration of these two elements in terms of K, L, M, N shell. Predict whether these are metallic or non-metallic.
Answer:
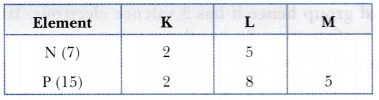
Both are non-metals as they tend to form bonds by gaining electrons.