NH3 and NF3 which has higher dipole moment and why?
Dipole moment of NH3 is higher than NF3 molecule.
- This is due to the fact that dipole moment is a vector quantity and hence resultant dipole moment in a molecule will depend upon the direction of dipole moments of individual bonds.
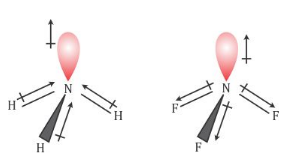
- Nitrogen has a lone pair of electrons and is more electronegative than hydrogen. So, in case of NH3, the orbital moment due to the lone pair of electrons on nitrogen is in the direction of lone pair of electrons is in the same direction of the 3 N-H dipole s.
- Thus, the molecule has a net dipole moment and it is polar.
- In NF3, fluorine being more electronegative than nitrogen pulls the shared pair of electrons of the N-F bonds towards itself. Because of this, the orbital moment due to lone pair of electrons and the resultant dipole moment of 3 N-F bonds point in the opposite directions to that of lone pair of electrons. This lowers the dipole moment of NF3.