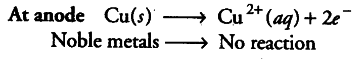Name the common elements present as anode mud in the electrolytic refining of copper. Why are they so present?
Metals which are less reactive and valuable as silver, gold, platinum, etc., are fo.und below anode as anode mud.
The reason is that being less reactive, they do not lose electrons at anode and collect under the anode as anode mud.
Copper metal at anode loses electrons in a usual manner to form {{Cu}^{2+}} ions.