NaH+H2O=NaOH+ H2 which type of redox reaction is this? Pls explain in detail
NaH+H2O → NaOH+ H2 is a type of displacement redox reaction.
Explanation:
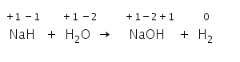
In the above reaction, Hydrogen of NaH is changing its oxidation state from -1 to +1 and Hydrogen from water changes from +1 to 0.
Hence NaH is reducing agent and H2O is the oxidizing agent.