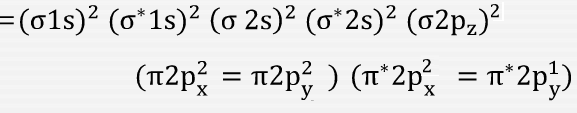More stable among O2+ and O2-
O2+ is more stable than O2-.
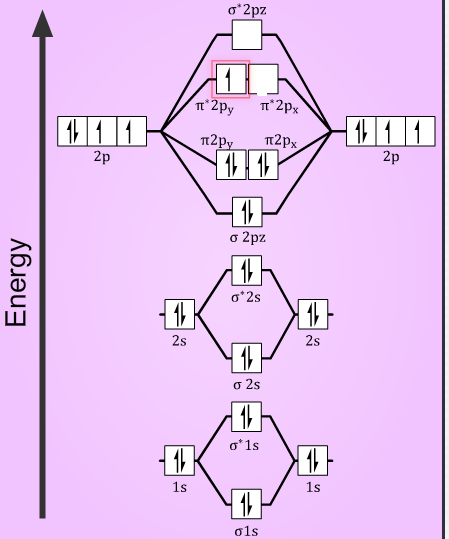
Reason: According to molecular orbital theory O2+ has 15 electrons &it has one electron in antibonding orbital.
molecular orbital diagram of O2+
Electronic configuration of O2+

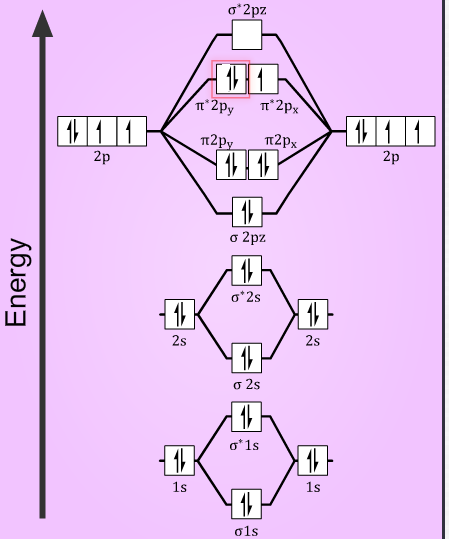
In the case of O2- 17 electrons are present &3 electrons are present in antibonding orbitals. If number of electrons more in antibonding orbital the molecule become unstable.
molecular orbital diagram of O2-
Electronic configuration of O2-