Fluorine reacts with ice and results in the change ${ H }{ 2 }$O +
${ F }{ 2 }$ ---------> HF + HOF
Justify that this reaction is a redox reaction.
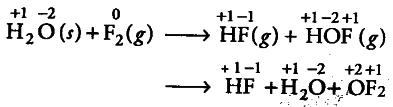
Oxidation number of F decreases from zero (in { F }_{ 2 }) to — 1 (in HF) and of O increases from — 2 to + 2
(in { OF }_{ 2 }). This shows that
{ F }_{ 2 } is reduced. It is not a disproportionation reaction, but only a redox reaction. In a disproportionation reaction, an element in one oxidation state is simultaneously reduced and oxidised.