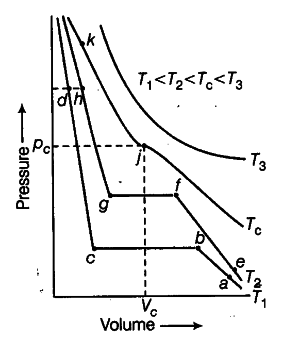
Isotherms of carbon dioxide at various temperatures are represented in the figure. Answer the following questions based on the figure.
(i) In which state will CO_{ 2 } exist between the points a and b at - temperature T_{ 1 }
(if) At what point will CO_{ 2 } start liquefying when temperature is T_{ 1 }
(iii) At what point will CO_{ 2 } be completely liquefied when temperature is T_{ 2 }
(iv) Will condensation take place when the temperature is T_{ 3 }?
(v) What portion of the isotherm at T_{ 1 } represent liquid and gaseous CO_{ 2 } at equilibrium?