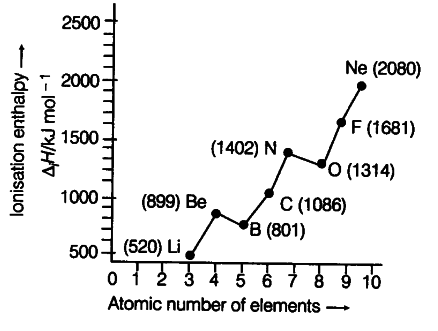
Ionisation enthalpies of elements of second period are given below.
Ionisation enthalpy/kcal {{mol}^{-1}} : 520, 899, 801,1086,1402,1314,1681, 2080.
Match the correct enthalpy with the elements and complete the graph given in figure. Also write symbols of elements with their atomic number.
We know that as we move from left to right across a period, the ionisation enthalpy keeps on increasing due to increased nuclear charge and simultaneous decrease in atomic radius. However, there are some exceptions also.
(i) In spite of increased nuclear charge, the first ionisation enthalpy of B is lower than that of Be. This is due to the reason that in case of Be, the outermost electron lies in the 2s-orbital but in case of B, it is present in a 2p-orbital. Since, the electrons in 2s-orbital are more tightly held by the nucleus than those present in 2p-orbital, therefore, ionisation enthalpy of B is lower than that of Be.
(ii) The first ionisation enthalpy of N is higher than that of O though the nuclear charge of O is higher than that of N. This is due to the reason that in case of N, the electron is to be removed from a more stable exactly half filled configuration ({{1s}^{2}} {{2s}^{2}} {{2px}^{1}} {{2py}^{1}} {{2pz}^{1}}) but in case of O ({{1s}^{2}} {{2s}^{2}} {{2px}^{2}} {{2py}^{2}} {{2pz}^{2}}), it is not so. Therefore, the first ionisation enthalpy of N is higher than that of O. The symbols of elements along with their atomic numbers are given in the graph.