Indicate whether or not each of the following structures is considered to be aromatic.
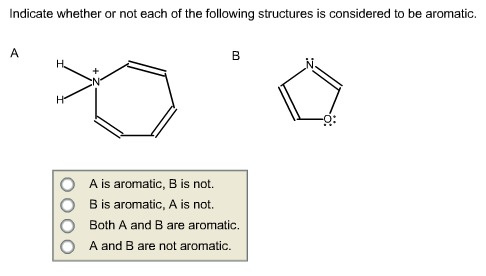
Answer:
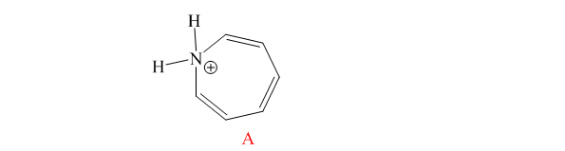
The compound has 611 electrons. So it obeys (4n+2) electrons. But there is no planer in the
structure. Hence, it is not aromatic.
The compound has 611 electrons. So it obeys (4n+2) It electrons. There is planer in the
structure. Hence, it is aromatic.

Hence, both compound B is aromatic and A is not.