In the ground-state electron configuration of Fe^{3+}, how many unpaired electrons are present?
Express your answer numerically as aninteger.

Part B
Build the orbital diagram for the ion mostlikely formed by phosphorus.
Use the buttons at the top of thetool to add sublevels. Click within an orbital to addelectrons.
Concepts and reason
The electrons are filled in the orbitals according to Hund’s rule. It states that pairing of electrons takes place only after all the degenerate orbitals are half-filled.
If an atom loses electrons, it gets positive charge and if the atom gains electrons, it gets negative charge.
Fundamentals
Ground state is the energy state of the molecule when all the electrons are present in lowest possible molecular orbitals.
In excited state, one electron goes from lower energy orbital to higher energy molecular orbital.
Answer:
Part A
The atomic number of Fe is 26.
It loses 3 electrons to form ![]() ion.
ion.
Hence, the number of electrons in ![]() is 23.
is 23.
Therefore, its ground state electron configuration is,
![]()
Write the orbital configuration for the outermost 3d orbital as follows:
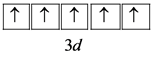
Hence, the number of unpaired electrons in ![]() are 5,
are 5,
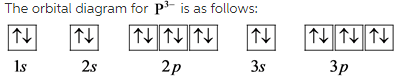
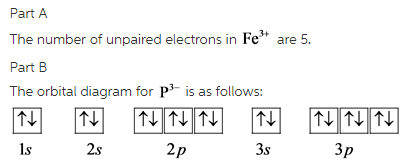
Part A
The number of unpaired electrons in ![]() are 5.
are 5.
In the 3d sub shell, there are 5 degenerate d orbitals. Hence, according to Hund’s rule, each orbital gets one electron and a total of 5 unpaired electrons.
Part B
The most likely ion formed by phosphorous is ![]() .
.
The electron configuration of ![]() is,
is,
![]()