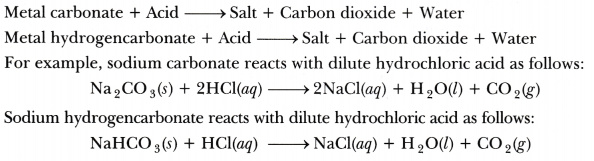
In the following schematic diagram for the preparation of hydrogen gas as shown in figure, what would happen if following changes are made?
In place of zinc granules, same amount of zinc dust is taken in the test tube.
Instead of dilute sulphuric acid, dilute hydrochloric acid is taken.
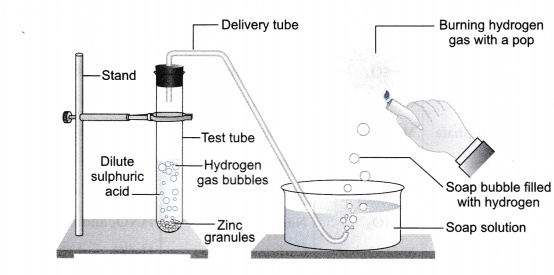
Sodium hydroxide is taken in place of dilute sulphuric acid and the tube is heated.
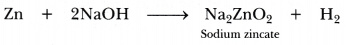
(ii) How do metal carbonates and metal hydrogencarbonates react with acids?
Answer:
-
Hydrogen gas will evolve with greater speed.
-
Almost same amount of gas is evolved.
-
If sodium hydroxide is taken, hydrogen gas will be evolved.
-
All metal carbonates and hydrogencarbonates react with acids to form a corresponding salt, carbon dioxide and water.