In the first excited state of hydrogen atom, its radius is found to be 21.2 x ${ 10 }^{ -11 }$ m. Calculate its Bohr radius in the ground state. Also calculate the total energy of the atom in the second excited state.
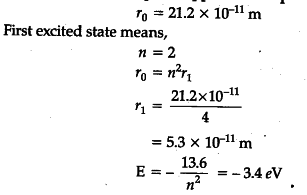
In the first excited state of hydrogen atom, its radius is found to be 21.2 x ${ 10 }^{ -11 }$ m. Calculate its Bohr radius in the ground state. Also calculate the total energy of the atom in the second excited state.
