In electrolysis of water, why is the volume of gas collected over one electrode is double that of gas collected over the other electrode.
The balanced chemical equation for the electrolysis of water is
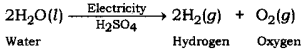
It can be observed from the balanced chemical equation that the volumes of hydrogen and oxygen produced during electrolysis are in the ratio 2:1. Thus, the volume of hydrogen gas collected in the process is double than that of oxygen.