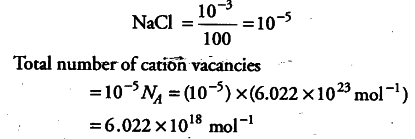Due to the addition of Sr$C{{l}_{2}}, each S{{r}^{2+}} ion replaces 2{{Na}^{+}} ions, but occupies only one lattice point in place of two Na+ ion. This creates cation vacancy. Number of moles of cation vacancies in 100 moles of NaCl is equal to {{10}^{-3}}$ mole.
Number of moles of cation vacancies in 1 mole of