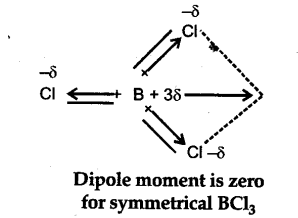
(1) B$C{{l}{3}} molecule due to s{{p}^{2}}$ hybridization of boron is trigonal planar molecule and has a symmetrical shape.
(2) Individual B-Cl bond has dipole, the molecule B$C{{l}{3}}$ has a zero dipole moment. This is due to the fact that individual dipole moments cancel out on account of the symmetry of the molecule
as shown below :