If ?= 2, what can you deduce about n?
If m? = 3, what can you say about ??
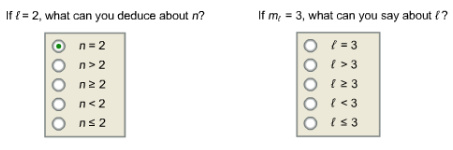
Answer:
The azimuthal quantum number, l equals to 2 represents d-orbital. The minimum value of principal quantum number, n for d-orbital is 3 as 1d and 2d orbital are not possible.
The value of principal quantum number is greater than 2.