Identify the type of chemical reaction taking place
(i) on mixing a solution of potassium chloride with silver nitrate, an insoluble white substance is formed.
(ii) on heating green coloured ferrous sulphate crystals, reddish-brown solid is left and smell of a gas having odour of burning sulphur is observed.
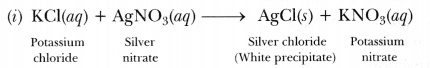
This reaction is an example of double displacement and precipitation reaction in which a precipitate of silver chloride is obtained.

This reaction is an example of decomposition (thermal decomposition) reaction because a single substance (FeS04) breaks down into three substances (Fe203, S02 and S03). Flere, S02 gives the smell of burning sulphur.