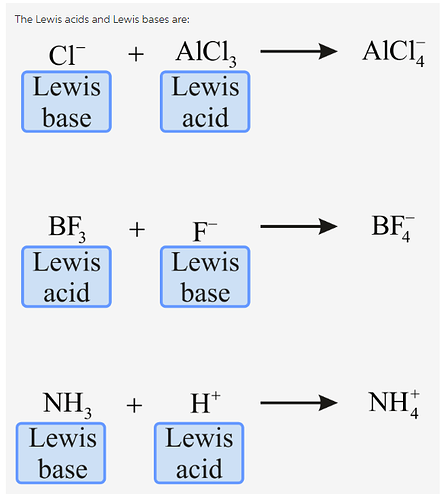
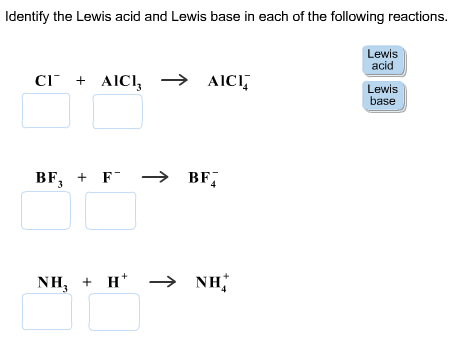
Identify the Lewis acid and Lewis base in each of the following reactions.
Concepts and reason
The concepts used to solve this problem are Lewis acid and Lewis base.
Cations and species with incomplete octet of electrons come under the category of Lewis acids.
Anions, lone-pair containing species and electron rich ![]() , all come under the category of Lewis bases.
, all come under the category of Lewis bases.
Fundamentals
According to Lewis theory of acid-base reactions, a Lewis acid is a chemical species (ion or molecule) having the capacity of accepting a pair of nonbonding electrons. A Lewis acid must have vacant orbitals to be able to accept electrons. For example,![]() is a Lewis acid because it has empty orbitals and hence can accept more electrons.
is a Lewis acid because it has empty orbitals and hence can accept more electrons.
A Lewis base is a chemical species (ion or molecule) having the capacity of donating a pair of nonbonding electrons. For example, ![]() is a Lewis base because it has lone pair of electrons which it can donate.
is a Lewis base because it has lone pair of electrons which it can donate.
Answer:
Consider the following reaction:
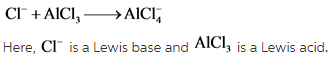
Consider the following reaction:

Consider the following reaction:
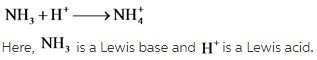
Explanation: