Identify the compound of calcium which is yellowish white powder and is used for disinfecting drinking water. How is it manufactured? Write the chemical equation for the reaction involved. What happens when it is left exposed to air?
(i) The compound is bleaching powder and the chemical name is calcium oxy chloride and formula is CaOCL2
(if) It is manufactured when dry slaked lime is added to chlorine gas.
Ca(OH)2 + Cl2 → CaOCl2 + H2O.
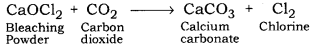
(iii) When it is left exposed to air it recats with the carbon dioxide in the air to produce chlorine gas.