Identify each of the following solids as molecular, ionic, or atomic.
CaCl2(s)
CS2(s)
B(s)
I2(s)
Concepts and reason
Matter exists in various forms such as gaseous state, liquid state, and solid state. In solid state, the constituent particles may be atoms, molecules, and ions that are held together by various types of forces of attractions such as van der Waals forces, dispersion forces, and electrostatic forces of attractions.
Fundamentals
Molecular solids:
The molecular solids are formed when molecules are held together by London dispersion forces, dipole–dipole forces, or hydrogen bonds. The molecular solids have lower melting points and poor conductors of electricity.
Ionic solids:
Ionic solids are formed when the positive ion and negative ion are held together by electrostatic force attractions. The ionic solids have high melting points and are good conductors of electricity.
Atomic solids:
Atomic solids are formed when atoms are bonded together by covalent attractions. The atomic solids are also called covalent-network solids. Atomic solids have high melting points and are poor conductors of electricity.
Answer:
The molecular solids are given below:
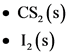
Explanation:
The molecular solids are carbon disulfide and iodine. The molecules present in the carbon disulfide are held together by dispersion forces and non-polar molecules. Iodine is a molecular solid in which molecules are held together by van der Waals dispersion forces.
The ionic solids are given below:
![]()
The molecular solids are given below:
![]()

Explanation:
Calcium chloride is an ionic solid that is formed between the ![]() ions. Boron is an atomic solid that is formed by covalent interactions between boron atoms.
ions. Boron is an atomic solid that is formed by covalent interactions between boron atoms.