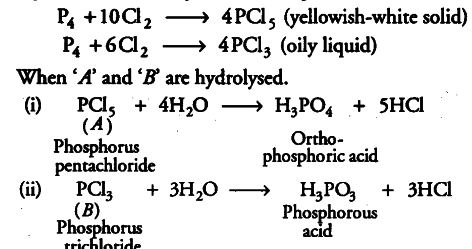
On reaction with $C{{l}_{2}}$, phosphorus forms two types of halides ‘A’ and ‘B’. Halide ‘A’ is yellowish-white powder but halide ‘B’ is colourless oily liquid.
Identify A and B, and write the formulae of their hydrolysis products.
Phosphorus on reaction with C{{l}_{2}} forms two types of halides A and B. Therefore, these halides must be PCI 5 and PCl 3. Since, halide A is yellowish-white solid but halide B is a colourless oily liquid thus, A is PCI 5 and B is PCl3.