(i) Give an example for a combination reaction which is exothermic.
(ii) Identify the oxidising agent and reducing agent in the following reaction:
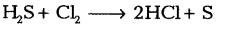
(iii) Name the phenomenon due to which the taste and smell of oily food changes when kept for a long time in open. Suggest one method to prevent it.
(i)
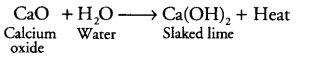
It is a combination reaction because two compounds on reaction give one product.
As heat is also evolved during the process, thus it is also an example of exothermic reaction.
(ii)
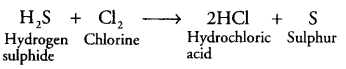
Oxidising agent - Cl2
Reducing agent - H2S
(iii) This is known as rancidity. Prevention Oily food must be kept in airtight containers.