(i) Arrange the following metals in decreasing order of their reactivity:
Fe, Zn, Na, Cu, Ag.
(ii) Show the formation of NaCl from Na and Cl atoms by the transfer of electrons.
(iii) Why do ionic compounds have high melting pomts?
(i) Na > Zn > Fe > Cu > Ag
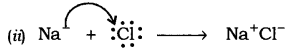
(iii) Ionic compounds have high melting points because of strong electrostatic forces of attraction.