(i) Account for the following:
- White silver chloride turns grey in sunlight.
- Brown coloured copper powder on heating in air turns into black coloured substance.
(ii) What do you mean by:
- Displacement Reaction?
- Reduction Reaction?
- Combination Reaction?
(i) 1. Silver chloride turns grey on exposure to sunlight because it decomposes in presence of sunlight to give silver (Ag).
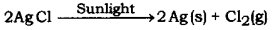
2. Oxidation of copper powder gives black colour copper oxide.
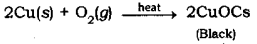
(ii) 1. Displacement reaction: A reaction in which a more reactive element displaces a less reactive elements from its salt sodium.
Example: Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
2. Redaction Reaction: Addition of hydrogen or removal of oxygen is called reduction.
Example: ZnO + C → Zn + CO
3.Combination Reaction: A reaction in which two or more reactants combine to give a single product.
Example: CaO(s) + H20(l) → Ca(OH)2(aq)