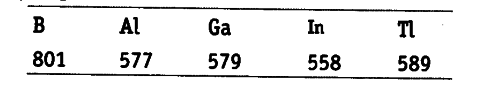
The first ionisation enthalpy values (in kJ / {{mol}^{2}})of group 13 elements are
How would you explain this deviation from the general trend?
The first ionisation enthalpy values (in kJ / {{mol}^{2}})of group 13 elements are

How would you explain this deviation from the general trend?
In general, on moving down the group (13th group) from B to Al, the ionisation enthalpy decreases with increase in atomic size and screening effect as expected. But ${ IE }_{ 1 }$ of Ga is slightly higher (only 2 kj ${{mol}^{-1}}$) than
${ IE }_{ 1 }$of Al. It is due to imperfect shielding of the valence electrons by 3d-electrons.
As a result of this, effective nuclear charge in Ga is slightly more than that of Al. That’s why (${ IE }_{ 1 }$ ) A ,H\ of Ga is slightly more than that of Al.
On moving from In to Tl,∆ i H1 of Tl is larger than that of In. It is due to the fact that effective nuclear charge outweighs the shielding effect of all the electrons present in '4d and 5d-electrons.