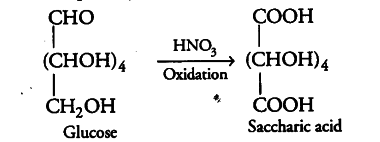
Glucose consists of 5 —OH groups. Among these, the —OH group present on the terminal carbon atom (i.e. C-6 atom) is called 1° hydroxyl group while all the four remaining —OH groups present on C-2, C-3, C-4 and C-5 atoms are called 2° hydroxyl groups. 1° hydroxyl groups are easily oxidised to carboxylic acid group while 2° hydroxyl groups undergo oxidation only under drastic conditions.
e.g. On oxidation with nitric acid, glucose gives a dicarboxylic acid, saccharic acid (also called glycaric acid) having the same number of carbon atoms as glucose. This indicates that glucose contains one primary (1°) alcoholic or hydroxyl group.