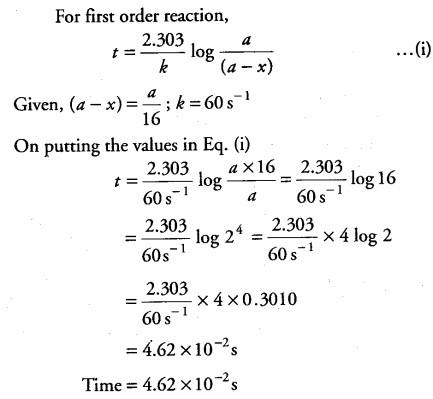The rate constant for a first order reaction is 60 ${{s}^{-1}}$. How much time will it take to reduce the initial concentration of the reactant to its l/16th value?
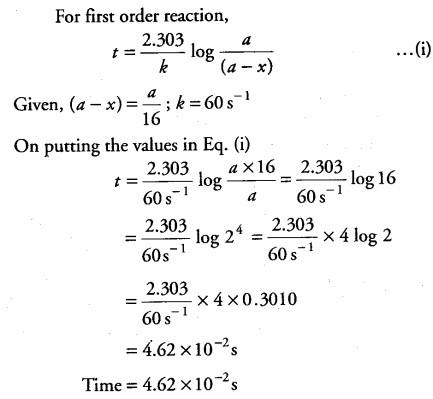
The rate constant for a first order reaction is 60 ${{s}^{-1}}$. How much time will it take to reduce the initial concentration of the reactant to its l/16th value?