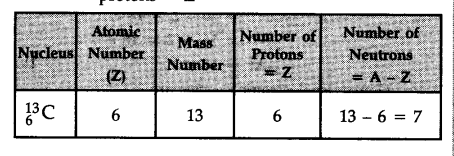
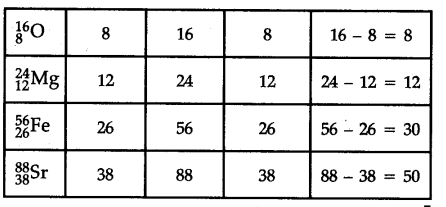
How many neutrons and protons are there in the following nuclei ?
![]()
The digit written as subscript represents the atomic numbers (Z) and that as superscript represents the mass number (A) of the element.
Number of neutrons = A - Z and number of protons = Z