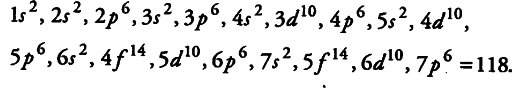How many elements can be accommodated in the present set up of the long form of the periodic table? Explain.
In the present set up of the long form of the periodic table, we have seven periods (i.e., principal quantum number, n = 7) and four blocks (s, p, dand f -block elements).
Therefore, the maximum number of elements which can be accommodated in the present set up of the long form of the periodic table in accordance with aufbau principle is