How is the electron configuration of ![]() .
.
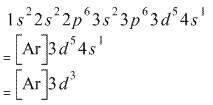
Concepts and reason
The concept of this question is that the filling of electrons in the shells and subshells of an atom takes place on the basis of Aufbau rule. It states that the subshells are arranged in increasing order of their n + l value where n is the number of main shell and l is the number of subshell.
Fundamentals
Some atomic connfigurations are exceptions to Aufbau rule. These are explained on the basis of the stability of subshells.
Answer:
The atomic number of Cr is 24. There are 6 electrons in the outer two shells of Cr that is, 3d and 4s.
The assumed electronic configuration of Cr is ![]()
Filling of electron take place in increasing order of n+l value of orbital.
For 3d orbital,
Substitute, 3 for n and 2 for l thus,

Th actual electronic configuration of Cr is ![]() . This is because half or full-filled orbitals are more stable than partially filled.
. This is because half or full-filled orbitals are more stable than partially filled.
The d subshell has five orbitals. This subshell is extremely stable when it is empty or half-filled. So, Cr exists as ![]() and not as
and not as ![]() to acquire half-filled configuration.
to acquire half-filled configuration.
Removing 3 electrons from Cr, Electronic configuration of ![]() .
.
Electronic configuration of ![]() .
.
To form a trivalent cation of Cr atom, 3 electrons are removed from its outer shells. One electron of the 4s shell and two electrons from the 3d shells.
