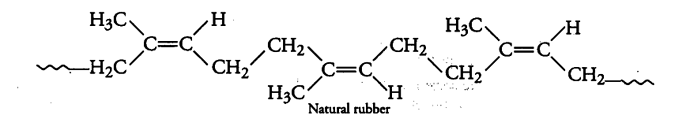
Natural rubber is cis-polyisoprene which is shown as:
The cis-configuration at double bonds does not allow the polymer chains to come closer for effective interactions and hence, intermolecular forces are quite weak. As a result, natural rubber (cis-polyisoprene) has a randomly coiled structure and hence shows elasticity.