How does a buffer resist change in pH upon addition of a strong acid?

Concepts and reason
Buffer solution is a solution which maintains its pH value on addition of small amount of strong acid or strong base into it. It is a solution which resists the change in pH.
Fundamentals
Acidic buffer solutions are the solutions which have a pH less than 7. These solutions are made from weak acid and one of its salts.
Basic buffer solutions are the solutions which have a pH greater than 7. These solutions are made from weak base and one of its salts.
Answer:
When a strong acid is added to buffer solution then conjugate base present in buffer solution takes its hydronium ion converting it into weak acid of conjugate base and water.
![]()
Due to this, amount of weak acid increases and amount of conjugate base decreases.
Explanation:
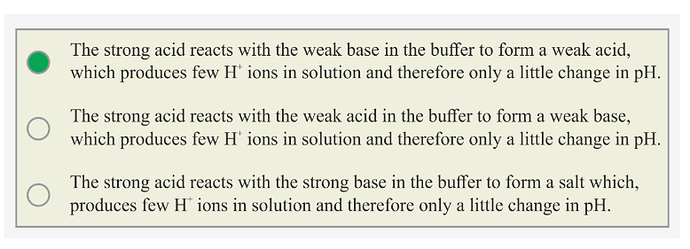
When a strong acid is added to the buffer solution then it reacts with weak base in the buffer to form a weak acid.
By adding strong acid in buffer solution, amount of weak acid increases. There will be a little change in pH.
Explanation:
Amount of weak acid is increased by adding strong acid in the buffer solution. Since the acid is weak. So, it produces only a few ![]() ions in the buffer solution and pH of the buffer decreases by very small amount.
ions in the buffer solution and pH of the buffer decreases by very small amount.