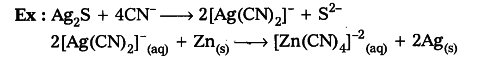- Metals at bottom of the activity series are often found in free state.
- The oxides of these metals can be reduced to metals by heat alone and sometimes by displacement from their aqueous solutions.
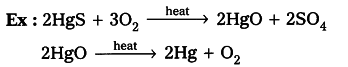
- When cinnabar is heated in air, it is converted into HgO, then reduced to mercury on further heating.
- Displacement from aqueous solution: When Ag2S is dissolved in KCN solution, it forms dicyanoargentate ions. When these ions are treated with Zn dust powder then Ag is precipitated.