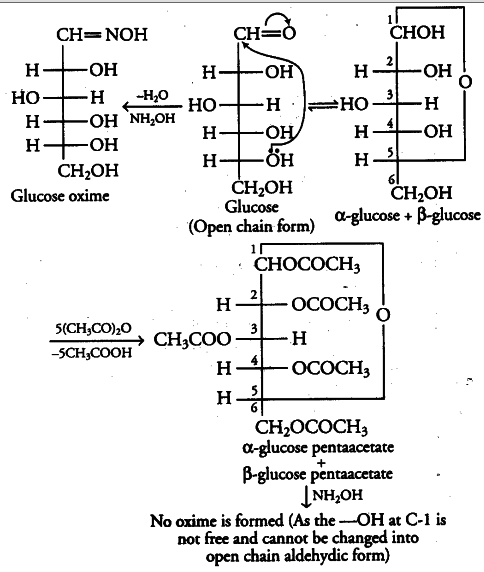
Aldehydic group can be tested by using hydroxyl amine reagent which forms oxime. At C-l position, the cyclic hemiacetal form of glucose contains an — OH group which gets hydrolysed in the aqueous solution to form the open chain aldehydic form which then reacts to form the corresponding oxime.
Hence, glucose contains an aldehydic group. Pentaacetate of glucose does not contain a free —OH group at C-1 position.
Therefore, in aqueous solution it cannot get hydrolysed to form the open chain aldehydic form and thus it does not react to form glucose oxime. Hence, glucose pentaacetate does not contain the aldehyde group.