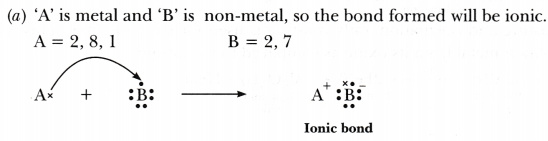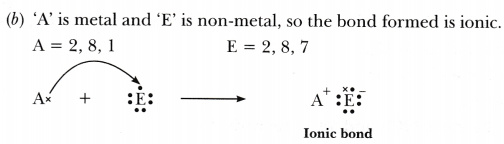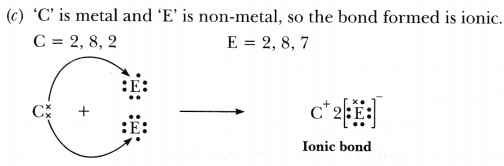- How do you classify elements into metals and non-metals on the basis of their electronic configuration? Choose metal and non-metal out of the following:
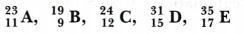
- What type of bond will be formed if
(a) ‘A’ combines with ‘B’?
(b) ‘A’ combines with ‘E’?
© ‘C’ combines with ‘E’?
(d) ‘D’ combines with ‘E’?
Answer:
- Elements which contain 1 to 3 electrons in their outermost shell are metals.
Elements containing 4 to 7 electrons in their valence shell are non-metals.

Hence A and C are metals whereas, B, D and E are non-metals. -
Type of bonds