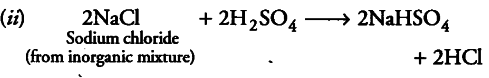How do you account for the following observations?
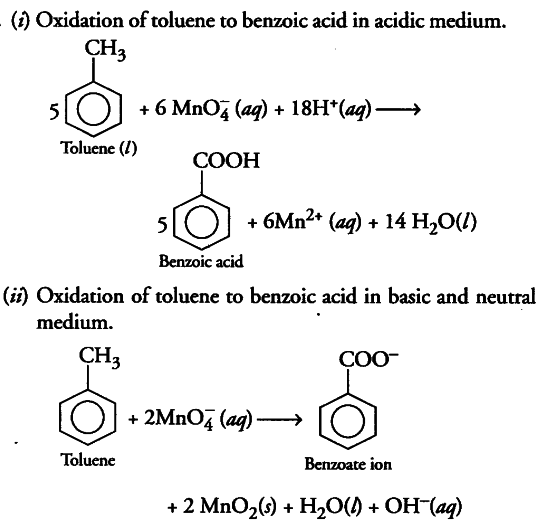
(i) Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why? Write a balance redox equation for the reaction.
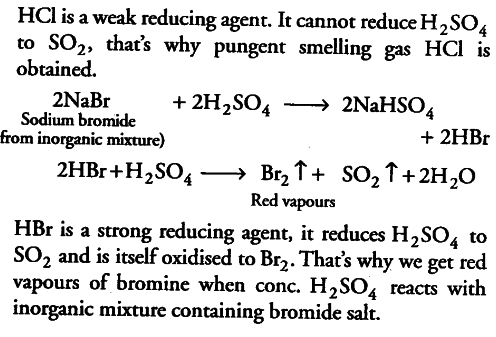
(if) When concentrated sulphuric acid is added to an inorganic mixture containing chloride, we get colourless pungent smelling gas HCl, but if the mixture contains bromide, then we get red vapour Of bromine. Why?
On industrial scale, alcoholic potassium permanganate is preferred to acidic or alkaline potassium permanganate because in the presence of alcohol, both the reactants KMn{ O }_{ 4 }^{ } and C_{6}H_{5}CH_{3} are mixed very well and form homogeneous solution and in homogeneous medium reaction takes place faster than in heterogeneous medium. Further more in neutral medium, OH“ ions are produced in the reaction itself.