Knowing the electron gain enthalpy values of -------»0~andO-and O---------> O2- as - 141 and
702 kJ mol-1 respectively, how can you account for the formation of a large number of oxides having O2 - species not O-?
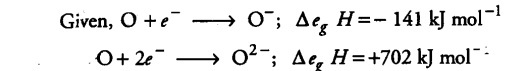
As it is obvious from the given data, the formation of divalent anion needs more energy as compared to monovalent anion where energy is released. Yet the number of oxides with - 2 oxidation state of oxygen is more evident.
The reason is that their crystal lattice is more stable due to stronger electrostatic forces of attraction involving divalent oxygen than the oxides in which oxygen is monovalent in nature.