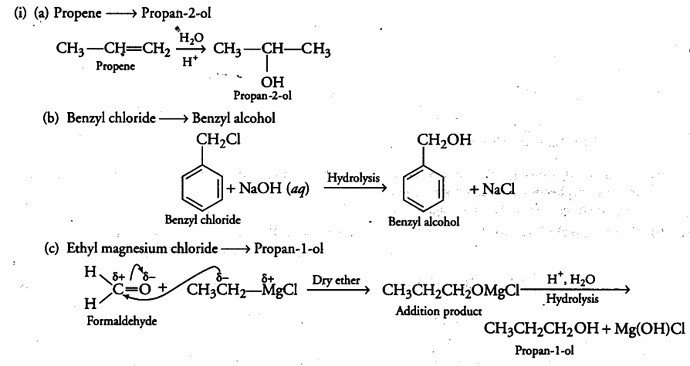
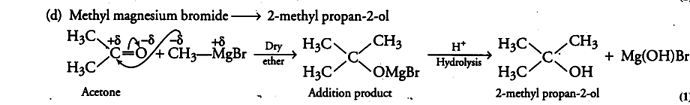
(i) How are the following conversions carried out?
(a) Propene --------»Propan-2-ol
(b) Benzyl chloride —> Benzyl alcohol
© Ethyl magnesium chloride —>Propan-1-ol
(d) Methyl magnesium bromide —» 2-methyl propan-2-ol.
(ii) Explain why propanol has higher boiling point than that of butane?