How are the following aspects of a reaction affected by the addition of a catalyst?
Concepts and reason
The concept that is used to solve the given problem is the property of the catalyst. Catalysts are the molecules that tend to affect the rate of a reaction significantly. However, it should be noted that catalyst itself does not undergo any significant change.
Fundamentals
The catalyst is the chemical compound that increases the rate of a reaction. Catalysts are usually the substances that are not consumed in the chemical reaction and thus, undergoes the least change.
Answer
The different aspects of a reaction are:
1.The activation energy of the forward reaction.
2. The activation energy of the backward reaction.
3.The rate of the forward reaction.
4.The rate of the backward reaction.
Since it is known that catalyst affects the activation energy by lowering it down, one might only consider the rate of forward reaction, spiking the backward reaction’s rate. However, one must be careful for both.
Part 1
A catalyst increases the rate of the forward reaction as well as the rate of the backward reaction. Apart from this, it also reduces the activation energy of both forward and backward reaction.
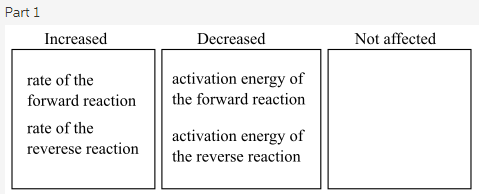
A catalyst increases the rate of a reaction. A catalyst does not specify in which direction the reaction would be accelerating. However, a catalyst can either accelerate the reaction rate in the forward or the backward direction, but never in both. A catalyst causes the decrease in the activation energy for both forward and backward reaction to accelerate them in the specific direction.
On might get confused in the increase and decrease of the rate of reaction in the forward and backward direction. In order to avoid such mistakes, one must be conceptually sound with the different properties of the catalyst.
Part 2
If one considers different aspects, of which are mentioned in part 1, the mechanism of the reaction of catalyzed and un-catalyzed reaction would differ significantly.
The aspect differs in the catalyzed and un-catalyzed reaction is its reaction mechanism.
The addition of a catalyst to any reaction, decreases its activation energy, thus, accelerating the rate of the reaction. It does not start the reaction; however, it reduces the amount of energy that is required to start a reaction. Further, in doing so, an intermediate product is formed in the catalyzed reactions.
It is important to be clear with the concept and mechanism of the catalyzed and un-catalyzed reaction. One might get confused in the reaction mechanism of either of the reaction.