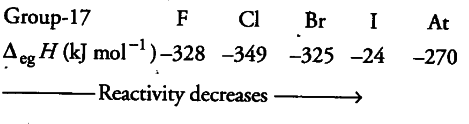The increasing order of reactivity among group 1 elements is Li < Na < K < Rb < Cs whereas that among group 17 elements is F > Cl > Br > I. Explain.
Chemical reactivity of alkali metals is exhibited by the loss of an electron leading to the formation of cation. The tendency to lose an electron depends upon the ionisation enthalpy and ionisation enthalpy decreases down the group. Hence, the reactivity increases down the group (Li < Na < K < Rb < Cs).
On the other hand, chemical reactivity of halogens is shown by the gain of an electron leading to the formation of anion. The tendency to gain an electron depends upon their electrode potentials. Their electrode potentials decrease from fluorine to iodine. Therefore, reactivity decreases down the group (F > Cl > Br > I). Furthermore, the tendency to gain an electron is also related to electron gain enthalpy.
Electron gain enthalpy becomes less and less negative as we move from chlorine to iodine. Hence, reactivity decreases from chlorine to iodine. Fluorine has less electron gain enthalpy but it is the most reactive due to its low bond dissociation enthalpy.